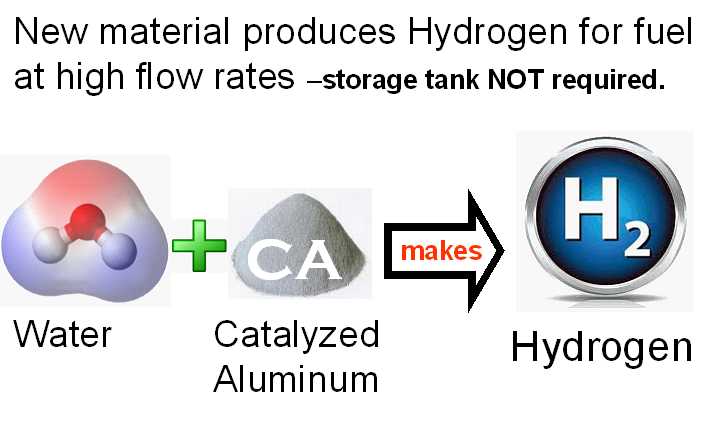
The unique thing about the new material is that it can produce hydrogen at any flow rate desired. That eliminates the need for storage tanks and lowers the cost of hydrogen fuel. One pint of the new CA material can produce hydrogen at a flow rate that is higher than that required to increase the MPG of an automobile by more than 32%.
The unique thing about the new material is that it can produce hydrogen at any flow rate desired. That eliminates the need for storage tanks and lowers the cost of hydrogen fuel. One pint of the new CA material can produce hydrogen at a flow rate that is higher than that required to increase the MPG of an automobile by more than 32%.